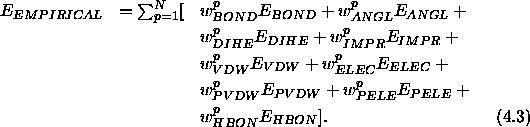
Empirical energy functions describe the energy of the molecule as a function of the atomic coordinates (Lifson and Stern 1982; Burkert and Allinger 1982; Brooks et al. 1983; Némethy, Pottie, and Scheraga 1983; Weiner et al. 1984; Karplus and Petsko 1990). Most of today's empirical energy functions contain conformational and nonbonded interaction energy terms involving sets of two, three, and four atoms. A harmonic approximation is used to account for deformations in bond length and angles. Four-atom terms are used for torsion potentials. Two-atom terms are employed for the nonbonded interactions. A variety of specific parameterizations for empirical energy functions are available in X-PLOR (Section 3.6).
XPLOR's empirical energy function has the general form
The sum is carried out over all double selections of atoms (see
Section 4.7) with weights 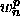 .
The default
for the double selections is one double selection involving
all atoms with unity weights.
The first four terms in Eq. 4.3
are conformational energy terms.
.
The default
for the double selections is one double selection involving
all atoms with unity weights.
The first four terms in Eq. 4.3
are conformational energy terms.
The remaining five terms in Eq. 4.3 describe
nonbonded interactions. The term
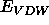 describes the non-symmetry-related van der Waals energy,
describes the non-symmetry-related van der Waals energy,
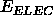 describes
the non-symmetry-related electrostatic energy,
describes
the non-symmetry-related electrostatic energy,
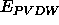 describes the van der Waals energy
between symmetry-related atoms,
and
describes the van der Waals energy
between symmetry-related atoms,
and  describes the symmetry-related electrostatic energy.
The term
describes the symmetry-related electrostatic energy.
The term
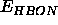 describes an explicit hydrogen-bonding energy. This
term is used only in older parameter files.
describes an explicit hydrogen-bonding energy. This
term is used only in older parameter files.
In the next sections, the empirical energy terms are described in more detail.