
describes the covalent bond energy where the sum is carried out
over all covalent bonds in the molecular structure selected
by the constraints interaction statement;
r is the actual bond length,
 is energy constants, and
is energy constants, and  is equilibrium constants
specified by parameter statements (Section 3.2.1).
is equilibrium constants
specified by parameter statements (Section 3.2.1).
The term
describes the bond angle energy where the sum is carried out
over all bond angles in the molecular structure selected
by the constraints interaction statement;
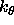 and
and  are energy constants and
are energy constants and
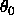 and
and 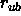 are equilibrium constants
specified by parameter statements (Section 3.2.1).
are equilibrium constants
specified by parameter statements (Section 3.2.1).
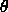 is the actual value of the angle, and
is the actual value of the angle, and 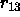 is the distance
between the first and the third atom defining the angle. The second
term in Eq. 4.5 is the Urey-Bradley term, which
is used by certain force fields (Burkert and Allinger 1982).
The default value for
is the distance
between the first and the third atom defining the angle. The second
term in Eq. 4.5 is the Urey-Bradley term, which
is used by certain force fields (Burkert and Allinger 1982).
The default value for 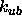 is zero.
is zero.
The angle between two planes, the first being defined through atoms i,j,k and the second through atoms j,k,l, is defined as a torsion angle where the atoms i,j,k,l are specified by the dihedral and improper statements (Section 3.1.1). The terms
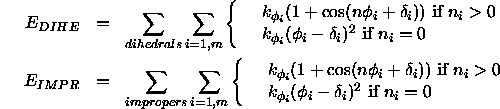
describe the dihedral and improper energy terms.
The sums are carried out
over all dihedral or improper angles in the molecular structure selected
by the constraints interaction statement;
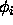 is the actual torsion angle,
is the actual torsion angle,
 are energy constants,
are energy constants,  are periodicities,
are periodicities,
 are multiplicities, and
are multiplicities, and  are phase shifts
(Section 3.2.1). Note that
the definition of dihedral and improper angles is identical.
However, X-PLOR maintains two separate topology and parameter
lists for dihedral and improper angles. Historically, improper
angles are mostly used with n=0 to maintain planarity or chirality,
whereas dihedral angles are used with n>0 to describe multi-minimum
torsion potentials.
are phase shifts
(Section 3.2.1). Note that
the definition of dihedral and improper angles is identical.
However, X-PLOR maintains two separate topology and parameter
lists for dihedral and improper angles. Historically, improper
angles are mostly used with n=0 to maintain planarity or chirality,
whereas dihedral angles are used with n>0 to describe multi-minimum
torsion potentials.
The specification of multiple dihedral or torsion angles with m>1 allows one to carry out a cosine expansion of a torsion potential for a particular instance involving four atoms or four atom types. Multiple dihedral angles and improper angles have to be indicated by using the MULTiple option both in the definition of the molecule's topology (Section 3.1.1) and in the specification of the corresponding parameter (Section 3.2.1). Internally, X-PLOR stores multiple dihedral or improper angles as multiple instances of the same combination of atoms or atom types.